Abstract
Aim
In this study, we investigated the co-occurring mutation landscape in acute myeloid leukemia (AML) patients with FLT3 internal tandem duplication (FLT3-ITD), and explored whether post-allogenic hematopoietic stem cell transplantation (allo-HSCT) sorafenib maintenance therapy could improve the outcomes of FLT3-ITD AML patients combined with other co-occurring genetic abnormalities.
Methods
A total of 456 FLT3-ITD AML patients receiving first allo-HSCT were included in this study. Gene mutations were detected using direct sequencing or next generation sequencing. Fusion genes were detected using a 53-gene polymerase chain reaction panel. The frequency of each genetic abnormality was investigated, and the mutation landscape was investigated. The primary outcome of this study was 3-year cumulative incidence of relapse. The secondary outcomes were 3-year overall survival (OS), disease-free survival (DFS) and non-relapse mortality (NRM). The outcomes were compared between patients who received post-HSCT sorafenib maintenance and those who did not in the whole population and in subgroups referring to co-occurring mutations.
Results
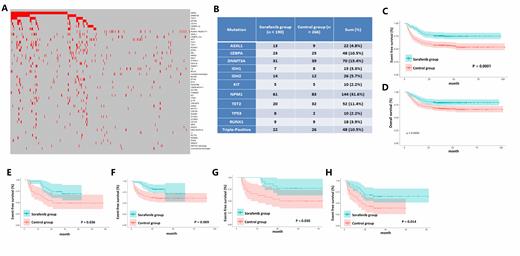
A total of 190 patients received post transplantation sorafenib maintenance therapy (sorafenib group) and 266 patients did not (control group). Of the patients receiving sorafenib pre-transplantation, the median duration of sorafenib therapy was 106 days (IQR 68 - 132) in the sorafenib group, and 99 days (IQR 71 - 142) in the control group (p = 0.883). Of the patients receiving post-transplantation sorafenib maintenance therapy, sorafenib was initiated at a median of 30 days (IQR, 30 - 52 days) after allo-HSCT, and continued for a median of 148 days (IQR, 112 - 150).
Median follow-up time was 38.7 months (IQR, 28.1 - 47.9). Thirty-four patients in the sorafenib group and 93 patients in the control group relapsed. The 3-year cumulative incidence of relapse was 18.0% (95% CI 13.1% - 24.3%) in the sorafenib group and 36.1% (95% CI 30.5% - 42.3%) in the control group (HR 0.43, 95% CI 0.29 - 0.64; p < 0.001). A total of 126 patients died, including 39 in the sorafenib group and 87 in the control group. Three-year DFS was 75.8% in the sorafenib group and 57.5% in the control group (HR 0.47, 95% CI 0.34 - 0.66; p < 0.001). Three-year OS was 79.5% for patients in the sorafenib group and 68.4% for patients in the control group (HR 0.57, 95% CI 0.39 - 0.83, p = 0.004).
Gene mutations were detected using a 12-mutation panel direct sequencing including FLT3-ITD, FLT3-TKD, NPM1, KIT, DNMT3A, CEBPA, ASXL1, TP53, TET2, IDH1, IDH2 and RUNX1 in all patients, and a 127-gene panel new generation sequencing in 188 patients.
Except FLT3, a total of 920 co-occurring gene mutations and 147 cytogenetic abnormalties were detected. The co-occurring mutations that presented in at least 10% the cases were NPM1 (31.6%), DNMT3A (15.4%), TET2 (11.4%), and CEBPA (10.5%). There were 10.5% patients presented with both NPM1 and DNMT3A mutations (Triple-mutated AML patients), and 13.8% patients combined with at least one additional genetic abnormality classified as adverse according to the 2017 ELN risk stratification other than FLT3-ITD. Cytogenetic abnormalities presented in more than 1% the patients were RUNX1-RUNX1T1, which occurred in 23 patients (5.0%), followed by +8 (2.9%), complex karyotype (2.6%), CBFB-MYH11 (2.4%), DEK-NUP214 (1.3%) and -y (1.3%).
Patients combined with NPM1(p = 0.009 and 0.009), DNMT3A (p = 0.036 and 0.086), triple-mutated AML patients (p = 0.030 and 0.027), and patients with at least one additional adverse abnormality (p = 0.014 and 0.005) benefit significantly in DFS and OS from post-transplantation sorafenib maintenance, but not those with CEBPA (p = 0.669 and 0.576) or TET2 (p = 0.375 and 0.178) mutations.
Conclusion
Post transplantation sorafenib maintenance therapy can improve the prognosis of FLT3-ITD AML patients combined with DNMT3A or NPM1, patients with triple-mutated AML, and patients combined with at least one additional adverse abnormalities, but not of those combined with CEBPA or TET2 mutations.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal